Clinical Trials Market to Reach USD 112.9 Bn by 2035, Expanding at a CAGR of 5.2% | Transparency Market Research
Clinical Trials Market expanding steadily with increasing focus on precision medicine and global drug development
Clinical Trials Market poised for strong growth driven by rising R&D investments and accelerating demand for innovative therapies.”
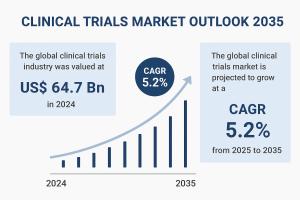
WILMINGTON, DE, UNITED STATES, September 29, 2025 /EINPresswire.com/ -- The global clinical trials market is poised for sustained growth through the next decade, underpinned by rising chronic disease prevalence, novel therapy development, and strong regulatory support. Valued at US$ 64.7 billion in 2024, the market is projected to expand at a CAGR of 5.2% from 2025 to 2035, surpassing US$ 112.9 billion by the end of 2035.— Transparency Market Research
The need for effective treatment modalities, growing pharmaceutical R&D budgets, and adoption of hybrid and decentralized trial models are catalyzing market expansion. Digital transformation, including AI-driven feasibility studies, electronic consent, eSource data capture, and synthetic control arms, is reshaping the way trials are designed and conducted.
Full Market Report available for delivery. For purchase or customization, please request here –
https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=78053
Market Overview
Clinical trials are mandated, systematic investigations to evaluate the safety and efficacy of new drugs, therapies, and devices. They are performed in sequential phases—Phase I (safety and dosing), Phase II (efficacy and side-effects), Phase III (large-scale efficacy vs. standard treatments), and Phase IV (long-term safety monitoring).
The market is broadly segmented by service type (protocol designing, patient recruitment & site identification, laboratory services, bioanalytical testing, supply & logistics, data management, patient engagement services, and others) and therapeutic area (cardiovascular, neurological, oncology, metabolic, respiratory, autoimmune, pain management, infectious diseases, and others).
Phase III trials continue to dominate the landscape, as they generate pivotal data required for regulatory approval and commercialization.
Analyst Viewpoint
According to TMR analysts, the clinical trials market is being shaped by shorter timelines, rising complexity of novel therapies, and emphasis on patient-centricity. Sponsors are increasingly outsourcing to CROs for end-to-end capabilities, spanning site selection, pharmacovigilance, logistics, and regulatory affairs.
Key structural shifts include:
Adoption of decentralized and hybrid trial models combining telemedicine, home healthcare, and community sites.
Use of master protocols (platform, basket, and umbrella designs) to evaluate multiple interventions under a single framework.
Deployment of AI-enabled site selection, patient recruitment, and real-time monitoring systems for efficiency.
Increased reliance on synthetic control arms and external data sources to minimize placebo exposure and accelerate approvals.
Key Drivers of Market Growth
1. Rising Prevalence of Chronic Diseases
The global burden of chronic illnesses such as diabetes, cancer, cardiovascular, and respiratory disorders is creating sustained demand for clinical trials. For example, the WHO reported that over 19.9 million new cancer cases were recorded worldwide in 2022. This rising disease burden is driving investments in innovative therapies and expanding clinical trial activity.
2. Increased Focus on Novel Therapies
Cell and gene therapies, biologics, immunotherapies, and RNA-based treatments are reshaping the trial landscape. As of September 2025, there are over 740 ongoing gene therapy trials and nearly 5,500 cell therapy trials globally. Such advanced modalities require specialized study designs, novel endpoints, and longer follow-up, fueling demand for clinical trial services.
3. Decentralized & Hybrid Operating Models
Decentralized models—integrating home visits, telemedicine, and wearable devices—are enhancing patient access, improving diversity, and reducing trial dropout rates. Hybrid trials are becoming a preferred model for balancing patient convenience with regulatory compliance.
4. Regulatory Support & Government Initiatives
Government agencies worldwide are offering funding, tax incentives, and expedited approval pathways for clinical trials, particularly in priority therapeutic areas like oncology, rare diseases, and infectious conditions.
Segment Analysis
By Service Type: Protocol designing, patient recruitment, laboratory services, bioanalytical testing, logistics, data management, and patient engagement dominate the service landscape.
By Therapeutic Area: Oncology leads the market, followed by cardiovascular and neurological disorders. Rising gene and cell therapy pipelines are expanding demand for specialized study protocols.
By Trial Phase: Phase III remains the largest contributor due to its strategic role in regulatory approval.
By Study Design: Interventional studies dominate, while observational and expanded access programs are gaining importance.
Regional Insights
North America: Maintains leadership with advanced healthcare infrastructure, FDA’s supportive regulatory framework, and high R&D spending. The U.S. is home to leading CROs, pharma companies, and biotech firms.
Europe: Strong presence in Germany, U.K., France, and Italy with harmonized regulatory guidelines and advanced research networks.
Asia Pacific: Poised for the fastest growth, driven by rising patient pools in China, India, and Japan, coupled with cost-efficient trial execution and government-backed initiatives.
Latin America & Middle East & Africa: Emerging markets with increasing trial activity, though challenges include regulatory delays and limited infrastructure.
Key Players
The market is competitive, with companies emphasizing AI integration, decentralized models, patient-centric services, and global site expansion. Key players include:
IQVIA Inc.
Labcorp
Syneos Health
Charles River Laboratories
Thermo Fisher Scientific Inc.
Parexel International Corporation
WuXi AppTec
Pfizer Inc.
ICON plc
Medpace
Velocity Clinical Research
Verily
ACM Global Laboratories
Invivoscribe, Inc.
PSI
BioAgile Therapeutics Pvt. Ltd.
Recent Developments
Merck (July 2025): Initiated EXPrESSIVE Phase 3 trial to assess MK-8527 for HIV pre-exposure prophylaxis across 16 countries.
Mural Health Technologies & ICON plc (March 2025): Partnership to deploy Mural Link platform for patient management, payments, and real-time site communications.
Opportunities and Challenges
Opportunities: Expansion of decentralized trial models, AI-powered site selection, growing real-world evidence demand, and untapped patient populations in emerging markets.
Challenges: High trial costs, complex protocols, recruitment delays, and cybersecurity risks in digitalized models.
Market Trends
AI and Big Data in Clinical Trials: Improving feasibility, recruitment, and analytics.
Outcome-Based Pricing Models: Sponsors and CROs aligning on performance guarantees.
Patient-Centric Trial Designs: Concierge services, simplified protocols, and digital engagement tools.
Integration of EHRs and Wearable Devices: Enabling seamless, real-time data capture.
Future Outlook
The clinical trials market is set to grow steadily, propelled by chronic disease burden, personalized therapies, and digital innovations. By 2035, decentralized and hybrid trial models, AI-powered decision-making, and regulatory reforms will reshape the competitive landscape. Companies investing in global reach, interoperable platforms, and patient-centric solutions will be well-positioned to capture significant market share.
Browse More Trending Reports:
Clinical Trial Management Systems Market - https://www.transparencymarketresearch.com/clinical-trial-management-systems-market.html
Clinical Trials Outsourcing Market - https://www.transparencymarketresearch.com/clinical-trials-outsourcing-market.html
Virtual Clinical Trials Market - https://www.transparencymarketresearch.com/virtual-clinical-trials-market.html
Clinical Trial Packaging Market - https://www.transparencymarketresearch.com/clinical-trial-packaging-market.html
About Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants use proprietary data sources and various tools & techniques to gather and analyses information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact:
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA – Canada Toll Free: 866-552-3453
Website: https://www.transparencymarketresearch.com
Email: sales@transparencymarketresearch.com
Follow Us: LinkedIn| Twitter| Blog | YouTube
Atil Chaudhari
Transparency Market Research Inc.
+1 518-618-1030
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.