Next Generation Antibody Therapeutics Market to Reach $22.88 Billion by 2033, Driven by Clinical Trial Successes
Immuno-oncology demand and FDA approvals drive next-gen antibody growth, positioning North America as the global leader.
Strong R&D pipelines, clinical trial success, and government biologics funding are accelerating the antibody therapeutics market worldwide.”
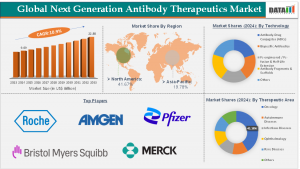
AUSTIN, TX, UNITED STATES, September 20, 2025 /EINPresswire.com/ -- According to DataM Intelligence, the next generation antibody therapeutics market size reached US$ 9.09 Billion in 2024, up from US$ 8.28 Billion in 2023, and is projected to reach US$ 22.88 Billion by 2033. The market is expected to grow at a CAGR of 10.9% during the forecast period (2025–2033). Among product categories, bispecific antibodies and ADCs hold the largest share due to their breakthrough performance in oncology and immune-related diseases. Regionally, North America dominates the global market, fueled by strong biopharmaceutical R&D, frequent FDA approvals, and widespread adoption of novel therapies, while the Asia-Pacific region is witnessing the fastest growth due to expanding biologics manufacturing, government initiatives, and rising healthcare investments.— DataM Intelligence
The next generation antibody therapeutics market represents one of the most dynamic and rapidly evolving areas within the biopharmaceutical industry. Unlike conventional monoclonal antibodies, these advanced therapeutics are engineered with enhanced specificity, better efficacy, and reduced immunogenicity. They include bispecific antibodies, antibody-drug conjugates (ADCs), Fc-engineered antibodies, antibody fragments, and other innovative platforms designed to treat complex diseases such as cancer, autoimmune disorders, and infectious diseases. With the increasing demand for targeted therapies and immuno-oncology treatments, next generation antibodies are transforming the therapeutic landscape and providing promising clinical outcomes for patients.
𝗚𝗲𝘁 𝗮 𝗦𝗮𝗺𝗽𝗹𝗲 𝗣𝗗𝗙 𝗕𝗿𝗼𝗰𝗵𝘂𝗿𝗲 𝗼𝗳 𝘁𝗵𝗲 𝗥𝗲𝗽𝗼𝗿𝘁 (𝗨𝘀𝗲 𝗖𝗼𝗿𝗽𝗼𝗿𝗮𝘁𝗲 𝗘𝗺𝗮𝗶𝗹 𝗜𝗗 𝗳𝗼𝗿 𝗮 𝗤𝘂𝗶𝗰𝗸 𝗥𝗲𝘀𝗽𝗼𝗻𝘀𝗲): https://www.datamintelligence.com/download-sample/next-generation-antibody-therapeutics-market
Key Highlights from the Report
➤ The global next generation antibody therapeutics market reached US$ 9.09 Billion in 2024 and is projected to hit US$ 22.88 Billion by 2033.
➤ The market is expected to grow at a CAGR of 10.9% during the forecast period 2025–2033.
➤ Bispecific antibodies and ADCs dominate the product segment due to high clinical success in oncology.
➤ North America remains the largest market, supported by advanced infrastructure and regulatory approvals.
➤ Asia-Pacific is the fastest-growing region, driven by rising biologics production and R&D investments.
➤ Key players are focusing on Fc-engineering and antibody fragments to enhance therapeutic safety and efficacy.
Recent Developments:
United States: Recent Developments in Next-Generation Antibody Therapeutics
1. In June 2025, the U.S. FDA approved Merck’s monoclonal antibody clesrovimab (Enflonsia) as a single-dose preventive treatment against RSV for infants up to one year old, regardless of birth weight.
2. In September 2025, Eli Lilly announced a US$5 billion investment to build a new manufacturing facility in Virginia dedicated in part to producing advanced therapies including antibody-drug conjugates (ADCs), to boost domestic capacity and resilience.
Japan: Recent Developments in Next-Generation Antibody Therapeutics
1. In March 2025, Japan’s Ministry of Health, Labour and Welfare approved TIVDAK® (tisotumab vedotin), the first and only antibody-drug conjugate (ADC) for advanced or recurrent cervical cancer that has progressed after chemotherapy.
2. In December 2024, the Ministry approved HYQVIA®, a facilitated subcutaneous immunoglobulin (fSCIG) therapy, for patients with agammaglobulinemia or hypogammaglobulinemia. This is Japan’s first and only fSCIG option, allowing reduced dosing frequency.
3. In September 2023, Japan approved EPKINLY™ (epcoritamab), the first bispecific T-cell engaging antibody treatment for relapsed or refractory large B-cell lymphoma (LBCL), including DLBCL, after two or more lines of systemic therapy.
Company Insights
Key players operating in the next generation antibody therapeutics market include:
• Amgen Inc.
• Pfizer Inc.
• Regeneron Pharmaceuticals
• AstraZeneca PLC
• Genmab A/S
• F. Hoffmann-La Roche Ltd.
• Novartis AG
• Takeda Pharmaceutical Company Limited
• AbbVie Inc.
• MacroGenics, Inc.
Market Segmentation:
The next generation antibody therapeutics market can be segmented based on product type, therapeutic application, and end-user.
By Product Type:
Bispecific Antibodies are leading the market, offering dual targeting ability for better treatment precision.
Antibody-Drug Conjugates (ADCs) are rapidly gaining adoption, especially in oncology, as they deliver cytotoxic drugs directly to tumor cells.
Fc-Engineered Antibodies are being designed for enhanced immune system activation and better therapeutic outcomes.
Antibody Fragments and Others are used for improved tissue penetration and reduced side effects.
By Therapeutic Application:
Oncology is the largest segment, with next generation antibodies showing strong success in hematologic malignancies and solid tumors.
Autoimmune and Inflammatory Diseases represent another major application area, driven by rising prevalence of rheumatoid arthritis, lupus, and multiple sclerosis.
Infectious Diseases are emerging as a focus area, especially in light of global viral outbreaks and antimicrobial resistance.
By End-User:
Hospitals and Specialty Clinics dominate due to widespread adoption of advanced antibody therapeutics in cancer care.
Research Institutes and Biopharmaceutical Companies play a vital role in clinical trials and antibody discovery programs.
Looking For A Detailed Full Report? Get it here: https://www.datamintelligence.com/buy-now-page?report=next-generation-antibody-therapeutics-market
Regional Insights:
North America holds the largest market share, primarily due to the presence of global pharmaceutical leaders, advanced clinical trial networks, and strong support from regulatory bodies like the FDA. The U.S. accounts for the majority of revenues, with companies such as Amgen, Regeneron, AbbVie, and Pfizer leading the charge in bispecific antibody and ADC development.
Europe represents the second-largest region, with strong biologics manufacturing hubs in Germany, Switzerland, and the UK. The European Medicines Agency (EMA) continues to support accelerated approvals, particularly for oncology-related antibody therapeutics.
Asia-Pacific is the fastest-growing region, fueled by government incentives for biologics production, rising cancer prevalence, and growing collaborations between Western pharma and regional biotech firms. Countries like China, India, South Korea, and Japan are investing heavily in antibody development, positioning the region as a global biopharma innovation hub.
Latin America and the Middle East & Africa are smaller markets but show increasing potential due to rising healthcare infrastructure, expanding access to biologics, and improving regulatory frameworks.
Market Dynamics:
Market Drivers
The primary driver of the next generation antibody therapeutics market is the rising global burden of cancer and autoimmune diseases, which require advanced, targeted therapies. The market is also benefiting from advances in antibody engineering technologies, growing clinical trial success rates, and strong R&D funding from pharmaceutical companies. Increasing government support for biologics and the expansion of immuno-oncology research are further accelerating growth.
Market Restraints
High manufacturing costs, complex development processes, and regulatory hurdles remain significant restraints. Producing bispecifics and ADCs requires advanced platforms, making them costly compared to conventional biologics. Limited awareness in low- and middle-income countries and potential safety concerns related to novel mechanisms of action also restrict adoption.
Market Opportunities
Opportunities lie in emerging markets such as Asia-Pacific, where biologics production capacity is rapidly expanding. The growing shift toward personalized medicine, AI-based antibody discovery, and rare disease treatments is opening new revenue streams. Furthermore, technological advances in computational antibody design, next-gen sequencing, and combination immunotherapies are creating breakthrough opportunities for industry players.
Get Customization in the report as per your requirements: https://www.datamintelligence.com/customize/next-generation-antibody-therapeutics-market
Reasons to Buy the Report
✔ Detailed analysis of market trends, size, and growth drivers.
✔ Coverage of emerging innovations like bispecific antibodies and ADCs.
✔ Regional breakdown with forecasts up to 2033.
✔ Insights into strategies of top market players.
✔ Evaluation of opportunities in oncology, autoimmune diseases, and rare disorders.
Frequently Asked Questions (FAQs)
◆ How big is the global next generation antibody therapeutics market?
◆ Who are the key players in the next generation antibody therapeutics market?
◆ What is the projected growth rate of the next generation antibody therapeutics market?
◆ What is the market forecast for next generation antibody therapeutics through 2033?
◆ Which region is expected to dominate the industry during the forecast period?
Conclusion
The next generation antibody therapeutics market is entering a transformative phase, driven by innovation, rising disease prevalence, and the push for precision medicine. With revenues projected to grow from US$ 9.09 Billion in 2024 to US$ 22.88 Billion by 2033, the market is poised for strong expansion. While high costs and regulatory complexities remain hurdles, the progress in bispecific antibodies, ADCs, and Fc-engineered antibodies highlights the industry’s ability to overcome challenges and deliver life-saving therapies. As North America continues to lead and Asia-Pacific emerges as a major growth hub, the future of next generation antibody therapeutics promises remarkable opportunities for patients, providers, and pharmaceutical innovators alike.
Request for 2 Days FREE Trial Access: https://www.datamintelligence.com/reports-subscription
Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
Competitive Landscape
Sustainability Impact Analysis
KOL / Stakeholder Insights
Unmet Needs & Positioning, Pricing & Market Access Snapshots
Market Volatility & Emerging Risks Analysis
Quarterly Industry Report Updated
Live Market & Pricing Trends
Import-Export Data Monitoring
Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg
Related Reports:
RNAi Therapeutics Market
Cancer Therapeutics Market
Sai Kiran
DataM Intelligence 4market Research LLP
877-441-4866
email us here
Visit us on social media:
LinkedIn
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.